Quick Links
Overview
The use of Remote Patient Monitoring (RPM) has skyrocketed as a way to help manage the health of individuals with chronic conditions while allowing them to stay safely at home. Many health conditions can be managed through RPM and remote physiologic monitoring, including diabetes, hypertension, congestive heart failure and even dementia. RPM uses technology to collect and transmit patient-generated health data and can work to support care management programs targeting populations or diagnoses.
RPM allows clinicians to track and manage data and can work to reduce complications and hospital readmissions. RPM puts patients at the center of their own care for better outcomes. It’s no surprise RPM usage has grown so quickly.
But with the broad use and rapid growth of RPM technologies comes the need for standards that serve as guardrails. How do patients, payers and clinical care teams know that the monitoring services they use are effective, safe, evidence-based and secure? That’s where URAC Remote Patient Monitoring Accreditation comes in – it allows organizations to demonstrate their excellence in technology-enhanced patient care management.
The URAC Difference
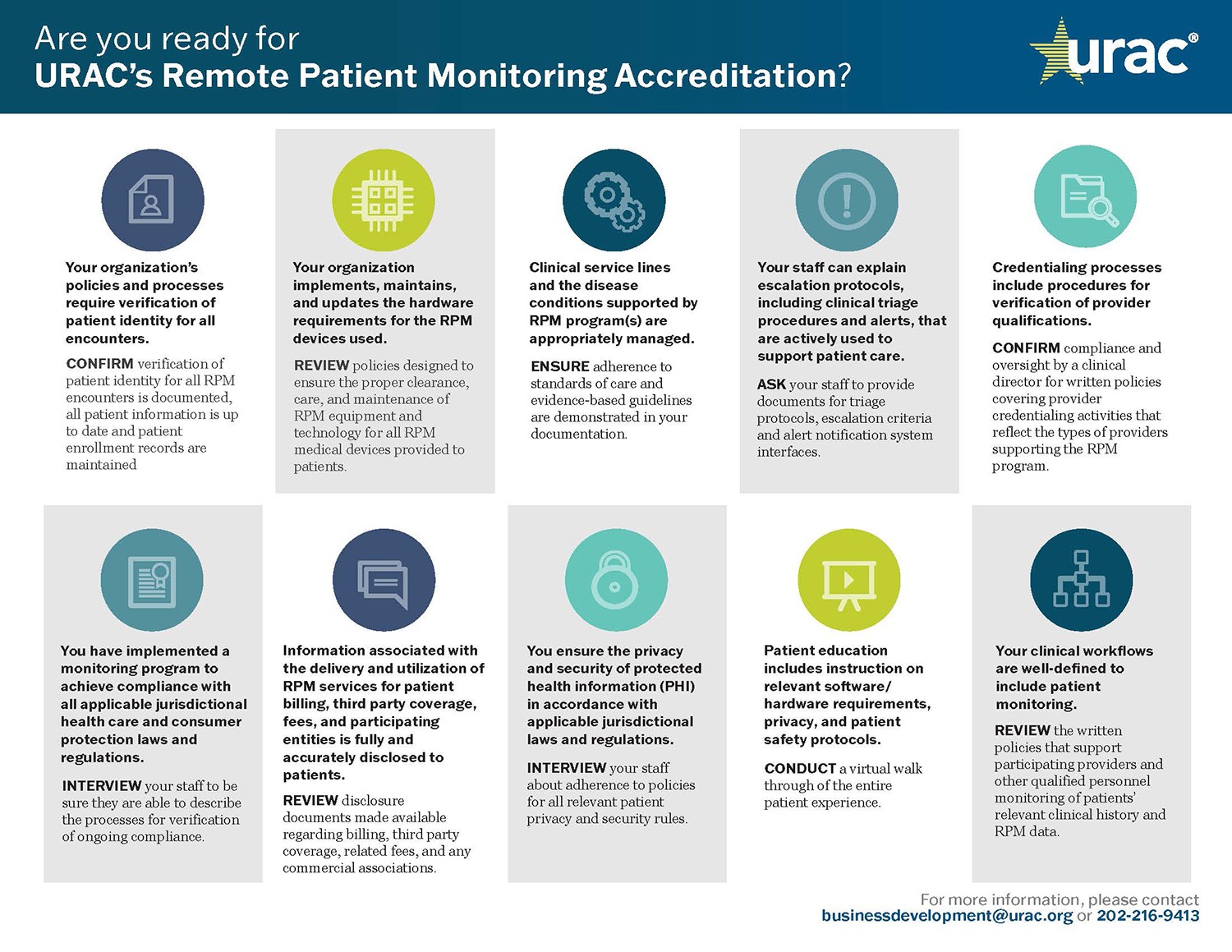
Although the U.S. Food and Drug Administration certifies RPM devices and several standards-setting bodies have developed security and related requirements, URAC’s Remote Patient Monitoring Accreditation Program is the first comprehensive set of standards created to assess many key aspects of an RPM program. The standards include:
- Operations
- Professional oversight
- Clinical workflow
- Quality and patient safety
- Technology requirements
- Risk management
There are three attributes that allow for URAC RPM Accreditation:
- The ongoing use of portable or wearable technology-enabled patient monitoring devices or patient home monitoring devices
- Secure transmission of patient data for assessment or monitoring
- Care facilitated outside traditional sites of care; patient and provider are not co-located
What to Expect
Within six months or less, URAC collaborates with your team to assess your approach and creates a flexible framework for continuous improvement. While URAC convenes experts to define the standards of excellence, they do not prescribe how your organization should meet those standards.
URAC’s approach stimulates innovation across the continuum of care through email, conference calls and educational webinars.
We award accreditation for a full three years. View our Remote Patient Monitoring Accreditation Standards-at-a-Glance for more information.
Who May Apply
RPM Accreditation standards speak directly to the provision of technology for patient care. To apply, an applicant must be a corporate entity that provides or supports RPM services.
This includes:
- Health systems
- Hospitals
- Health plans
- Disease management organizations
- Case management organizations
- Clinical management organizations
- Telehealth providers
This accreditation differs from URAC’s Telehealth Accreditation Program: RPM is focused on the digital communication link between the provider and the patient over time, telemedicine represents the actual virtual care encounter between the provider and the patient in addition to the two-way communication link.
Go here for more information on our Telehealth Accreditation Program.